Start up needs to be done quickly and right the first time.
Our Approach
effective recruiting takes experience, innovation, and determination.

Advertising:
- Traditional: fliers, chart review, community organizations
- Digital: Facebook, Twitter and Google
Website:
- Landing pages with lead tracking
- Chat, text and other innovative communication features
- Responsive and mobile designs
Pre-screening:
- Our full-time recruiters do nothing but recruit with inbound and outbound campaigns
- Recorded pre-screenings for quality assurance
- Innovative recruiting software
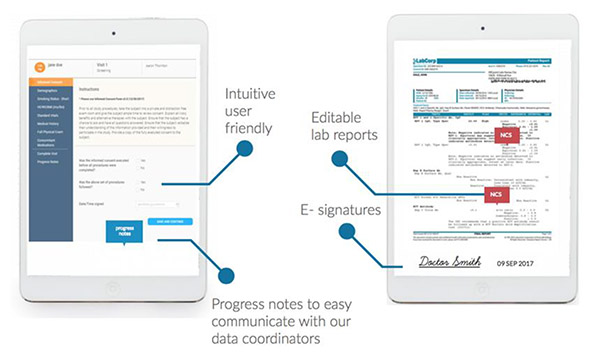
we collect source data electronically with a powerful all-in-one “esource” using software built specifically for clinical research sites.
- Source collected by tablet, no paper
- Quicker, more accurate and easily queried
- Monitored in real time, reducing errors
- Secure login, remote signatures, and audit ready
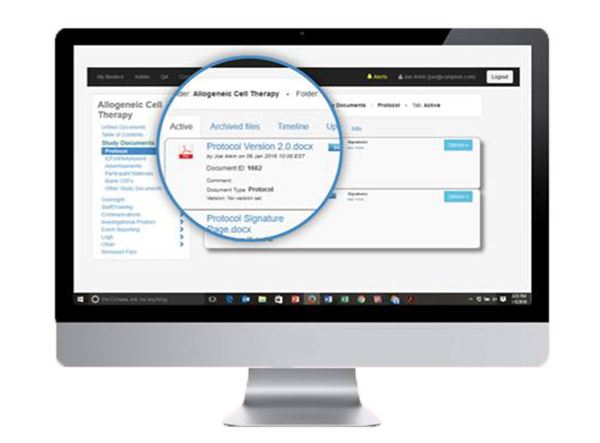
we use complion FOR OUR e-regulatory & document management. this cloud based platform is built specifically for sites and is accepted as the industry standard.
NO MORE PAPER BINDERS: Reduce costs, avoid redundancy and free up office space.
FEATURES: Easily archive from Outlook, obtain eSignatures and view files from your mobile device. Use eForms instead of printing and scanning.
AUDIT READY: Keep everything organized with a built-in audit trail for improved transparency. 21 CFR Part 11 compliant.
REMOTE MONITORING: Easy access to regulatory documents from any device at any time.

close